Clinoptilolite-Ca Mineral Data
General Clinoptilolite-Ca Information
Chemical Formula: (Ca,Na,K)2-3Al3(Al,Si)2Si13O36•12(H2O)
Composition: Molecular Weight = 2,742.13 gm
Potassium 1.50 % K 1.80 % K2 O
Sodium 1.48 % Na 1.99 % Na2 O
Calcium 2.78 % Ca 3.89 % CaO
Magnesium 0.15 % Mg 0.25 % MgO
Aluminum 6.61 % Al 12.49 % Al2 O3
Silicon 29.91 % Si 63.98 % SiO2
Hydrogen 1.74 % H 15.57 % H2 O
Oxygen 55.84 % O
______ ______
100.00 % 99.97 % = TOTAL OXIDE
Empirical Formula: Ca1.9 Na1.76 K1.05 Mg0.17 Al6.72 Si29.2 O72 •23.7(H2 O)
Environment: Most commonly formed as a devitrification product of silicic volcanic glass from tuffs. Also occurs in cavities in rhyolites, andesites, and basalts.
IMA Status: Approved IMA 1977 (Dana # Added)
Locality: Kuruma Pass, Fukushima Prefecture, Japan. Link to MinDat.org Location Data.
Name Origin: From the Greek klino - "oblique" ptylon - "feather" and lithos - "stone.." The Ca-dominant member of the clinoptilolite series.
Name Pronunciation: Clinoptilolite-Ca + Pronunciation
Clinoptilolite-Ca Image
Images:
Clinoptilolite-Ca
Comments: Druse of colorless, clear tabular somewhat pseudohexagonal crystals of clinoptiloite-Ca in vug.Location: Fish Creek Quarry, Clackamas River, Estacada, Clackamas County, Oregon, USA.
Scale: See Image.© Rruff Database
Clinoptilolite-Ca Crystallography
Axial Ratios: a:b:c =0.9831:1:0.4119
Cell Dimensions: a = 17.66, b = 17.963, c = 7.4, Z = 1; beta = 116.47° V = 2,101.39 Den(Calc)= 2.17
Crystal System: Monoclinic - Prismatic
X Ray Diffraction: By Intensity(I/Io ): 3.971(1), 8.99(0.85), 3.91(0.7),
Physical Properties of Clinoptilolite-Ca
Cleavage: {010} Perfect
Color: White, Reddish white.
Density: 2.1 - 2.2, Average = 2.15
Diaphaneity: Transparent to Translucent
Fracture: Uneven - Flat surfaces (not cleavage) fractured in an uneven pattern.
Habit: Crystalline - Fine - Occurs as well-formed fine sized crystals.
Habit: Tabular - Form dimensions are thin in one direction.
Hardness: 3.5-4 - Copper Penny-Fluorite
Luster: Vitreous (Glassy)
Streak: white
Optical Properties of Clinoptilolite-Ca
Gladstone-Dale: CI meas = 0.008 (Superior) - where the CI = (1-KPDmeas /KC ) calc = 0.017 (Superior) - where the CI = (1-KPDcalc /KC )PDcalc = 0.2239,KPDmeas = 0.226,KC = 0.2279
Optical Data: Biaxial (+/-), a=1.476-1.491, b=1.479-1.493, g=1.479-1.497, bire=0.0030-0.0060, 2V(Calc)=0-72, 2V(Meas)=31-48. Dispersion r > v to r < v strong.
Calculated Properties of Clinoptilolite-Ca
Electron Density: Bulk Density (Electron Density)=2.18 gm/cc
Fermion Index: Fermion Index = 0.01
Photoelectric: PEClinoptilolite-Ca = 1.89 barns/electronU=PEClinoptilolite-Ca x r electron = 4.11 barns/cc.
Radioactivity: GRapi = 21.89 (Gamma Ray American Petroleum Institute Units)
Concentration of Clinoptilolite-Ca per GRapi unit = 4.57 (%)
Estimated Radioactivity from Clinoptilolite-Ca
Clinoptilolite-Ca Classification
Dana Class: 77.01.04.02b (77) Tectosilicate Zeolite group
(77.01) true zeolites
(77.01.04) Heulandite and related species
77.01.04.01 Heulandite-Ca (Ca,Na)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 Mono
77.01.04.01a Heulandite-Na ! (Na,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 Mono
77.01.04.01b Heulandite-K ! (K,Na,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 2/m
77.01.04.01c Heulandite-Sr ! (Sr,Na,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 Mono
77.01.04.01d Heulandite-Ba ! (Ba,Ca,K,Na,Sr)5Al9Si27O72•22(H2O) C 2/m 2/m
77.01.04.02 Clinoptilolite-K (Na,K,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, C 2, Cm Mono
77.01.04.02a Clinoptilolite-Na ! (Na,K,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, C 2, Cm Mono
77.01.04.02b Clinoptilolite-Ca ! (Ca,Na,K)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m 2/m
77.01.04.03 Stilbite-Ca NaCa4[Al8Si28O72]•n(H2O)(n=28-32) C 2/m 2/m
77.01.04.03a Stilbite-Na ! Na3Ca3[Al8Si28O72]•n(H2O)(n=28-32) C 2/m 2/m
77.01.04.04 Stellerite CaAl2Si7O18•7(H2O) F mmm 2/m 2/m 2/m
77.01.04.05 Barrerite (Na,K,Ca)2Al2Si7O18•6(H2O) Amma or Ammm 2/m 2/m 2/m
Strunz Class: 09.GE.05 09 - SILICATES (Germanates)
09.G - Tektosilicates with Zeolitic H2O
09.GE -Chains of T10O20 Tetrahedra
09.GE.05 Clinoptilolite-Na ! (Na,K,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, C 2, Cm Mono
09.GE.05 Clinoptilolite-K (Na,K,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, C 2, Cm Mono
09.GE.05 Clinoptilolite-Ca ! (Ca,Na,K)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m 2/m
09.GE.05 Heulandite-Ca (Ca,Na)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 Mono
09.GE.05 Heulandite-K ! (K,Na,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 2/m
09.GE.05 Heulandite-Na ! (Na,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 Mono
09.GE.05 Heulandite-Sr ! (Sr,Na,Ca)2-3Al3(Al,Si)2Si13O36•12(H2O) C 2/m, Cm, C 2 Mono
09.GE.05 Heulandite-Ba ! (Ba,Ca,K,Na,Sr)5Al9Si27O72•22(H2O) C 2/m 2/m
Other Clinoptilolite-Ca Information
References: NAME( Duda&Rejl90) PHYS. PROP.(Enc. of Minerals,2nd ed.,1990) OPTIC PROP.(Dana8)
See Also: Links to other databases for Clinoptilolite-Ca : Am. Min. Crystal Structure Database Athena GeoScienceWorld Google Images Google Scholar MinDAT Mineralienatlas (Deutsch) Online Mineral Museum QUT Mineral Atlas Ruff.Info Search for Clinoptilolite-Ca using:
[AOL ]
[Bing ]
[Dog Pile ]
[GeoScienceWorld ]
[HotBot ]
[Ixquick ]
[Lycos ]
[MAMMA ]
[Scirus ]
[Teoma ]
[WebCrawler ]
[Wikipedia ]
[YAHOO ]
Visit our Advertisers for Clinoptilolite-Ca :
Ask about Clinoptilolite-Ca here : Ask-A-Mineralogist from the Mineralogical Society of AmericaMindat.org's Discussion GroupsOriginal Rockhounds Discussion GroupRockhounds Discussion Group on Yahoo GroupsMineral Discussion Forum from Fabre Minerals - also available in
Espa�ol
Print or Cut-and-Paste your Clinoptilolite-Ca Specimen Label here :
Clinoptilolite-Ca
(Ca,Na,K)2-3Al3(Al,Si)2Si13O36•12(H2O) Dana No: 77.01.04.02b Strunz No: 09.GE.05 Locality:
Notes:
Dakota Matrix
Excalibur Minerals
Exceptional Minerals
Hudson Insitute
John Betts Fine Minerals
Mc Dougall Minerals
Mineral News
Rock and Mineral Shows
Weinrich Minerals, Inc.



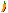 - barely detectable
- barely detectable